THE GROUP OF A THOUSAND COLUMNS
"The Group of a Thousand Columns" is a series of columns situated just outside the Temple of Warriors. It is known for the carvings of people, gods, animals, and serpents which are shown all around the structures.
THE REACTION AND RESULTS
Palladium Catalyzed Macrocyclization via Alkyne-Alkyne Coupling
Trost, B. M.; Dong, G. B. J. Am. Chem. Soc. 2010, 132, 16403-16416.
Trost and his group were excited to share a complex macrocyclization reaction that uses alkyne-alkyne coupling. The macrocyclization of Compound 21 varies in yield depending on the solvent it is reacted in. In benzene, it yields 22%. In THF, it yields a poor 23 % in 5 days. Finally, the Trost's team figured that the best results came from a toluene solvent where it yielded 56%. They even reasoned that in this special reaction, a small amount of reactant yields a higher percentage of product whereas a large amount of reactant yields a low percentage of product.
LEADING QUESTIONS
Palladium-Catalyzed Addition of Terminal Alkynes to Acceptor Alkynes
Trost, B. M.; Sorum, M. T.; Chan, C.; Ruehter, G. J. Am. Chem. Soc. 1997, 119, 698.
The team of Trost, Sorum, Chan, and Ruehter believe that using this mechanism (Pd-catalyzed addition) to achieve addition of terminal alkynes to acceptor alkynes is a practical and efficient way of organic synthesis that is not heavily limited to certain outcomes. Many similar reactions, depending on the reagents, create very specific types of products, whereas in this mechanism, various types of needed product may be obtained without side effects. This is due to the 3 phases of the mechanism:
(1) Activation of terminal alkyne C-H bond,
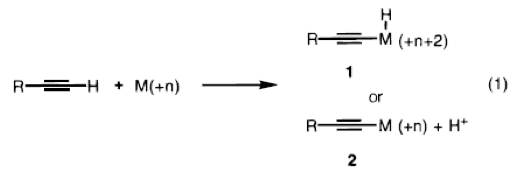
(2) Addition of pi-system to acceptor alkyne,
(3) Protonation of the vinyl palladium intermediate.
These three steps in conjugation with an electron-rich and sterically demanding ligand, such as TDMPP, allow for maintenance of an unsaturated palladium species in which a reaction occurs. Trost and his team believe that this chemoselective insertion to the C-H bond of the terminal alkyne works because of the sensitive nature of the pi system and the acidity of the proton. Furthermore, compounds that would normally produce unwanted byproducts such as malonates and alcohols (due to more acidic protons) do not interfere. Since the reaction only requires a 1:1 ratio of reactants to catalysts, it is highly efficient in yielding versatile products that have double and triple bonds, which can be selectively manipulated.